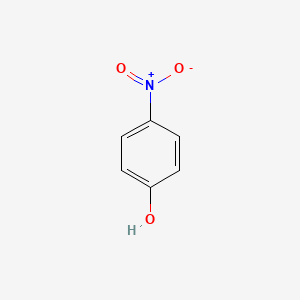
Para Nitrophenol Melting Point
So indirectly this question is asking about the melting point of Ortho and Para Nitro Phenol. 1138 deg C BP exp database.
Brown method Melting Pt deg C.
Para nitrophenol melting point. This does reflect the degree of intermolecular hydrogen bonding. This increases boiling point. This different behavior is due to having intramolecular hydrogen bonding.
Due to this reason para nitrophenol has higher boiling point than ortho nitro phenol. 279 deg C VP exp database. 4-aminophenol is an amino phenol one of the three possible isomers which has the single amino substituent located para to the phenolic -OH group.
Documentation regarding 4-Nitrophenol including CAS MSDS. It is a conjugate acid of a 2-nitrophenolate. 4578470 C at 760 mmHg Vapour Pressure.
Hene O-nitrophenol has a lower boiling point than P-nitrophenol. Or 2-nitrophenol was produced but they were not detected in the brain slices. Intermolecular hydrogen bonding leads to a molecular association.
000029 Modified Grain method MP exp database. Application 4-Nitrophenyl chloroformate can be used. The melting points and undoubtedly also the boiling points of the nitro phenols reflect the degree of intermolecular hydrogen bonding.
In intermolecular hydrogen bonding association of molecules take place but in intramolecular hydrogen bonding there is no such type of association between the molecules. P-nitrophenol has intermolecular hydrogen bonding. 324 Melting Point 205 to 208 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
1875 C 3695 F. For orthonitrophenol hydrogen bonding would be intramolecular ie. 1995 Boiling Pt Melting Pt Vapor Pressure Estimations MPBPWIN v142.
P-aminophenol was obtained as white solid melting point 167-690C as Wiyid nulls absorbs at 3350-3300cm. 26149 Adapted Stein. 2-nitrophenol is a member of the class of 2-nitrophenols that is phenol in which one of the hydrogens that is ortho to the hydroxy group has been replaced by a nitro group.
Compound Melting point Boiling point Water solubility at 25 C 2-Nitrophenol 43 45 C 215 C 2 g L 3-Nitrophenol 89 95 C 278 C 135 g L 4-Nitrophenol 113 114 C 279 C 16 g L. 12ml 01mol of acetic anhydride was warmed with the mixture stirred vigorously and warmed on a water bath. National Toxicology Program Chemical Repository Database.
It has a role as a metabolite and an allergen. Melting Point C 0 - 1006 101 - 20010. 4606 K Boiling point.
In the synthesis of 4-substituted phenyl derivatives of. 02072016 The melting points of ortho meta and para-nitrophenol. 979E-05 mm Hg at 20 deg C.
O-nitrophenol has intramolecular hydrogen bonding. 367 to 369 F NTP 1992. The nitrophenols have completely different physical behavior based on the position of nitro group.
Acylation of P-aminophenol About 11g 01mol of p-aminophenol was suspended in 30ml of water contained in a 250ml beaker. 7076 Mean or Weighted MP VPmm Hg25 deg C. Sigma-Aldrich offers a number of 4-Nitrophenol products.
Para Nitro Phenol is symmetric in nature as you can observe from its structure due to which its melting point increases and volatility decreases. The interactions between molecules are much stronger when there are intermolecular hydrogen bonds as in para-Nitrophenol because the bonds are formed between molecules. Boiling Pt deg C.
0012 mmHg at 25C Enthalpy of Vaporization. Increase in that order. 284 C 543 F.
This results in more stability and lower melting and boiling points. 75730 kJmol Flash Point. 2307293 C Index of Refraction.
As a reagent for the activation of amines alcohols and thiols. Ortho-Nitrophenol has a lower melting and boiling point than para-Nitrophenol. 4-Nitrophenol C6H5NO3 CID 980 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
Consider a typical example. So we can conclude Ortho. Melting point of any compound depends on its crystal lattice and and symmetric compound have high lattice energy.